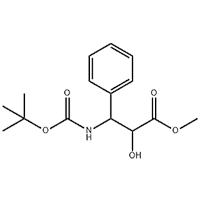
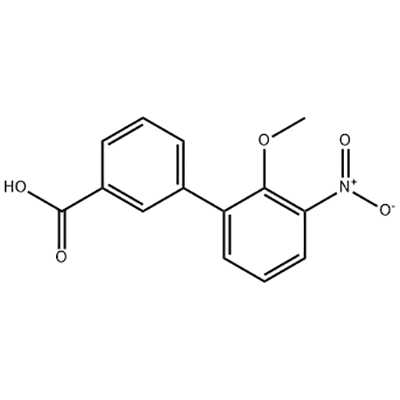
2'-Methoxy-3'-nitro-biphenyl-3-carboxylic acid
2'-Methoxy-3'-nitro-biphenyl-3-carboxylic acid
2'-Methoxy-3'-nitro-biphenyl-3-carboxylic acid ana amfani dashi azaman tsaka-tsakin Eltrombopag.
Eltrombopag, wanda GlaxoSmithKline (GSK) ya haɓaka a Burtaniya kuma daga baya aka haɓaka tare da Novartis a Switzerland, shine farkon kuma kawai wanda aka amince da ƙananan ƙwayoyin cuta marasa peptide TPO agonist a duniya.An amince da Eltrombopag ta FDA ta Amurka a cikin 2008 don maganin cututtukan thrombocytopenic purpura (ITP), kuma a cikin 2014 don maganin anemia mai tsanani (AA).Hakanan shine magani na farko da FDA ta Amurka ta amince don maganin AA a cikin shekaru 30 na baya-bayan nan.
A cikin Disamba 2012, FDA ta Amurka ta amince da Eltrombopag don maganin thrombocytopenia a cikin marasa lafiya tare da ciwon hanta na kullum C (CHC), don haka marasa lafiya na hepatitis C tare da rashin jin dadi saboda ƙananan platelet zasu iya farawa da kuma kula da interferon bisa daidaitattun maganin cututtuka na hanta.A ranar 3 ga Fabrairu, 2014, GlaxoSmithKline ya sanar da cewa FDA ta ba da nasarar cancantar maganin miyagun ƙwayoyi na Eltrombopag don maganin hemopenia a cikin marasa lafiya tare da anemia mai tsanani na sinadarai (SAA) waɗanda ba su da cikakkiyar amsa ga immunotherapy.A ranar 24 ga Agusta, 2015, FDA ta Amurka ta amince da Eltrombopag don maganin thrombocytopenia a cikin manya da yara masu shekaru 1 da haihuwa tare da thrombocytopenia na rigakafi na yau da kullum (ITP) waɗanda basu da isasshen amsa ga corticosteroids, immunoglobulins ko splenectomy.A ranar 4 ga Janairu, 2018, an amince da Eltrombopag da za a jera shi a kasar Sin don maganin thrombocytopenia na rigakafi na farko (ITP).



![pentamethylene bis [1- (3,4-dimethoxybenzyl) -3,4-dihydro-6,7-dimethoxy-1H-isoquinoline-2-propionate], dioxalate](http://cdn.globalso.com/jindunchem-med/image281-300x300.png)

![Casp ungin Acetate;Caspofungin acetate;Cancidas;Caspofungin acetate [USAN:BAN:JAN];](http://cdn.globalso.com/jindunchem-med/fbe17385-300x300.jpg)